The University of Nebraska-Lincoln (UNL) is committed to maintaining the highest ethical principles in the conduct of research with human subjects through the UNL Human Research Protection Program (HRPP).
UNL’s HRPP earned full accreditation from the Association for the Accreditation of Human Research Protection Programs (AAHRPP) in 2008 with ongoing re-accreditation occurring at various intervals since then but not greater than every five years. AAHRPP is a nonprofit organization that requires maintenance of rigorous standards to achieve this distinction. This voluntary accreditation process is a testament to UNL’s commitment to human subjects protections and how each member of the research community plays a role.
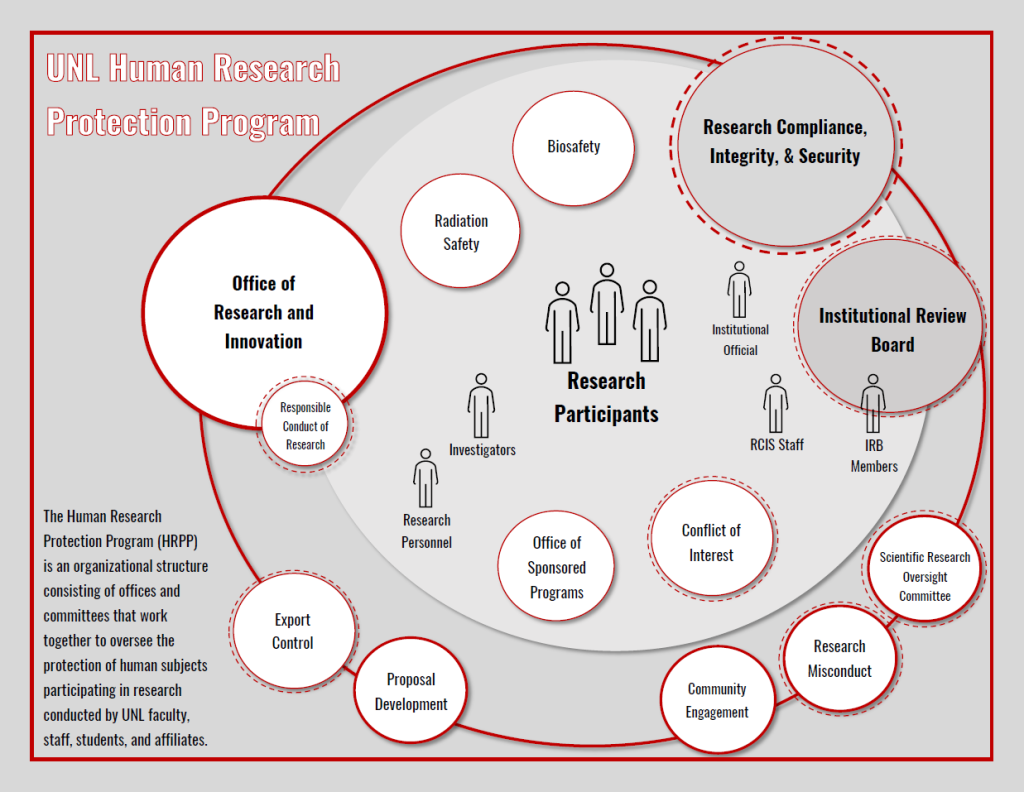
The HRPP is an organizational structure that includes the Institutional Review Board (IRB) and Research Compliance, Integrity, and Security. It also includes the Institutional Official who is responsible for the overall program, other compliance committees such as the Radiation Safety Committee and the Conflict of Interest in Research Committee, and the staff who manage the administrative oversight of the programs. Through these programs we all work together to oversee the protection of human subjects participating in research conducted by UNL faculty, staff, students and affiliates.
IRB Metrics
The metrics presented below include both real-time data for the current calendar year, as well as five years-worth of past calendar year data for comparison. Each of our three most insightful metrics are presented within their own tab. Click through to learn more about the work IRB coordinators are managing for all UNL faculty and student investigators. *Note: These metrics include outliers.
We are presently working on updating our daily metrics tracker and are working to get the tracker back up as soon as possible.
All applications to the IRB are submitted via NuRamp. UNL is AAHRPP accredited and a member of SMART IRB for multi-site research.
The Institutional Review Board (IRB) and the Human Research Protection Program (HRPP)
The UNL Institutional Review Board (IRB) operates within the Human Research Protection Program (HRPP) to ensure the protection of human subjects and the integrity of research within any given study. In order for this to occur, the IRB performs the review functions throughout the life of a project while the HRPP is a systems approach to how the institution oversees the implementation of the conduct of human subjects research.
The IRB is comprised of UNL faculty, staff and unaffiliated community members from differing disciplines with expertise based on UNL’s research portfolio. The IRB is charged by the Chancellor to “independently review and approve all human participant research conducted or supported by the faculty, students, staff, or other representatives of UNL . . . through sufficient resources and decisional autonomy,” documented with Policy #1.006: Authority Granted by UNL to the IRB Operating in the HRPP.
In order for a project to require oversight by the IRB it must meet the regulatory definitions of research AND human subjects. Policy #3.001: Investigational Activities Requiring IRB Review and Approval defines activities that must be reviewed and approved by the IRB before the project begins (including recruitment).
- Research is “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.”
- Human subject is a living individual about whom an investigator (whether professional or student) conducting research: (i) obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or (ii) obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.
The HRPP assists the IRB through several administrative functions while management of the HRPP is administered through Research Compliance, Integrity, and Security. The HRPP and IRB work closely together, but both have their own specific functions and responsibilities.
The HRPP and IRB’s most commonly performed processes are:
| HRPP | IRB |
| Composed of review committees and ancillary review processes (including, for example, the COI/COC Committee and Institutional Biosafety Committee), the Institutional Official, and staff supporting the administration of the IRB. | Composed of a specific number and composition of members representing the diverse interests and concerns of the University, the local community, and the state of Nebraska. |
| Facilitates the review process of all research projects. | Conducts initial and continuing review of research projects and votes to approve or disapprove projects. |
| Facilitates the review of all adverse events, noncompliance, unanticipated problems, reviews information, and oversees reporting to university and regulatory officials. | Reviews reportable new information such as adverse events, noncompliance, and unanticipated problems. |
| Facilitates the monitoring process of approved studies. | Monitors approved studies or appoints a neutral party to monitor studies. |
| Tracks all IRB-investigator project-specific conflicts of interest. | Reviews all IRB-investigator project-specific conflicts of interest in conjunction with the Conflict of Interest Committee (COI). |
| Performs project audits and reports results to the IRB. | Reviews audits performed by the HRPP. |
| Manages the AAHRPP accreditation process. | Ensures that UNL maintains accreditation through upholding institutional policies and maintaining high standards of human subjects research requirements. |
| Tracks all required human subjects training for research personnel and staff and conducts staff and investigator education. | Participates in initial and continuing education for IRB members. |
| Enters into and manages single IRB reliance agreements. | Reviews projects where UNL acts as the Reviewing IRB under a single IRB reliance agreement. |
| Verifies compliance with local and state laws, and federal laws and regulations. | Reviews projects in accordance with local and state laws, and federal laws and regulations. |
| Maintains SOPs related to human research protections for research projects. | Follows SOPs to ensure the protection of human subjects in research projects. |
| Manages HIPAA requirements for research projects. | Issues HIPAA authorization and waivers of consent for research projects. |
| Facilitates communication between the IRB and investigator(s) throughout the life of a project. | |
| Provides educational outreach, receives input from, and facilitates communication with research participants. |
If you have questions, concerns, complaints, or suggestions about human subjects research at the University of Nebraska-Lincoln, contact the IRB at 402-472-6965 or irb@unl.edu.








